Have you ever wondered how an ice melt works or what makes salt melt ice?…
Ice melt blends are rapidly becoming a growing segment of the ice melt market. But is there really more power in a salt blend? Why should you use a salt blend? And what makes a blend more powerful or effective than just using a straight ingredient like salt or calcium? Well, first, lets break it down and look at the three most common types of ice melt chemicals: Magnesium, Calcium, and Salt.
Magnesium
Magnesium chloride is greatly hydroscopic, meaning that water is attracted to it. Also, magnesium dissolving into water is an exothermic reaction, causing it to generate heat instantly on contact with ice and snow. This allows it to quickly start melting and dissolve into a brine. This powerful brine can melt effectively down to -15°F.
Magnesium is also a relatively safe and less corrosive chemical than other ice melters. Below are some of the benefits to using mag:
- Less irritating to the skin
- Less corrosive to metal surfaces
- Safer around vegetation
- Safer for use around animals and humans
- Environmentally friendly
Calcium
Calcium chloride is even more hydroscopic and exothermic than magnesium. This means that it generates even more heat when in contact with ice and snow. Because of the high heat, calcium is an exceptionally fast performing ice melt and can melt to an extreme temperature of to -25°F.
However, while the performance of calcium is exceptional, it is a harsh chemical, being highly corrosive and skin irritating. This makes it a poor choice for areas with pets and high foot traffic. The high heat can also damage new concrete if it has not been fully cured.
Salt
Salt, on its own, is a relatively weak ice melter and can only effectively melt down to around 5°F. But when salt is used with magnesium or another highly soluble substance, they help to increase the ion count in the melting brine allowing refreeze temperature to be lowered substantially.
The Power of Salt Blends
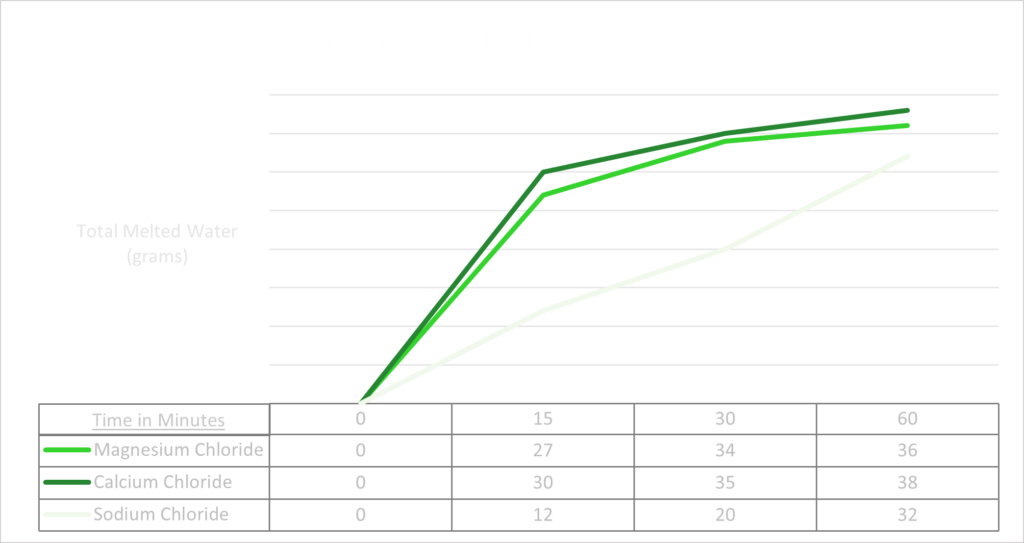
As this graph shows, magnesium and calcium melt very quickly. But after about an hour they have slowed down while the salt is still actively melting. After about an two hours, the magnesium and calcium will have completely stopped melting and the salt would still be working. Now obviously, this is only holds true in a storm where it keeps snowing and adding more ice/snow to melt.
While magnesium and calcium may each be more effective up-front than salt, they dissolve at a far higher rate than salt and over time require more product to melt the same amount of ice. Magnesium and calcium will melt fast, but with the help of salt they can also last longer and be more efficient. And while salt cannot melt by itself in the low temperatures of a winter storm, when mixed with magnesium or calcium it can be a powerful and long-lasting blend. Even though all three of these products work on their own, they each have their pros and cons. By creating a blend, the good elements of each product can be captured and turned into a premium ice melt.
I hope this has helped you to better understand the power behind salt blends and why they are more beneficial than simply putting down a straight product.
Check out our line of premium TruMelt Ice Melt blends for a high quality and long-lasting ice melt blend!

